The rapidly growing global e-mobility industry requires new, innovative flame retardants and demand keeps increasing massively
Compared to classic cars with combustion engines,
more
ECHA Suggestion of classification, labelling and also restriction of selected chlorinated flame retardants paves the way for an increased need for halogen free flame retardants
The European Chemicals Agency ECHA recently propos
more
The Working Group „Flame Retardants“ brings together many participants from the flame retardants value chain and celebrates Prof. Manfred Döring’s well-deserved retirement
Finally, the meeting of the working group “F
more
“ECOFRAM" addresses the need for more sustainable flame retardants and showcases developments from science and industry
The International Conference on Eco-Friendly Flame
more
RoHS: Impact study finds positive results, review process has started
The importance of RoHS, the restriction of hazardo
more
“Fire Resistance in Plastics" addresses the need for flame retardants for e-mobility – halogen-free solutions in clear focus
The Fire Resistance in Plastics is one of the most
more
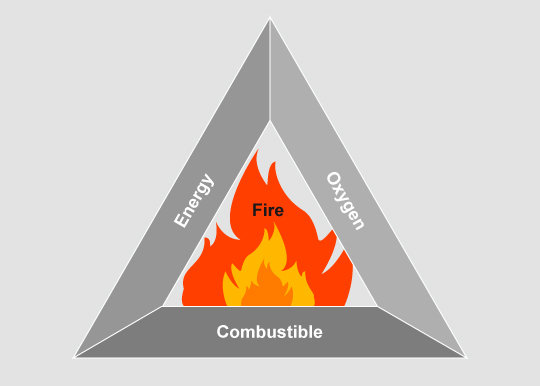
Fire initiation and combustion process
When does it burn?
The fire triangle demonstrates that three factors
- fuel
- heat
- air (oxygen)
must coincide in order for anything to burn.
The combustion process
The three factors mentioned above are related to each other in the combustion process: the combustion of plastics will be taken as an example in the following.
The combustion of plastics is a process comprising many steps and basically initiated by a heat source initiating the decomposition of the polymer. These two steps, heating and decomposition of the polymer consume energy (endothermic process) for overcoming the high binding energies between individual atoms (200 to 400 kJ/mole).
Combustible gases are formed, which mix the oxygen of the air and ignite. This leads to the exothermic part of the process, i. e. flame propagation and heat release. Pyrolysis of the polymer is reinfoced by thermal feedback (heat release), which fuels the flame at an increasing level. The flame is supported by extremely high energy H· and OH· radicals, which confer a high velocity to the flame.
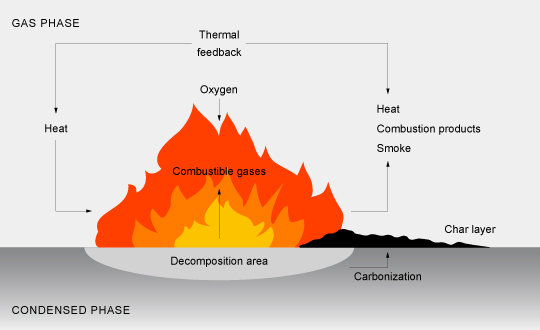
Flame retardants may inhibit the chemical reactions in the flame by removing the high energy radicals, or initiate the formation of a charred layer on the surface. Thus, the polymer is protected against the attack of heat and oxygen of the air and prevents the formation of flammable gas mixtures.